Influence of aging on corrosion resistance and structure of the passive film formed on Al?2Li binary alloys
-
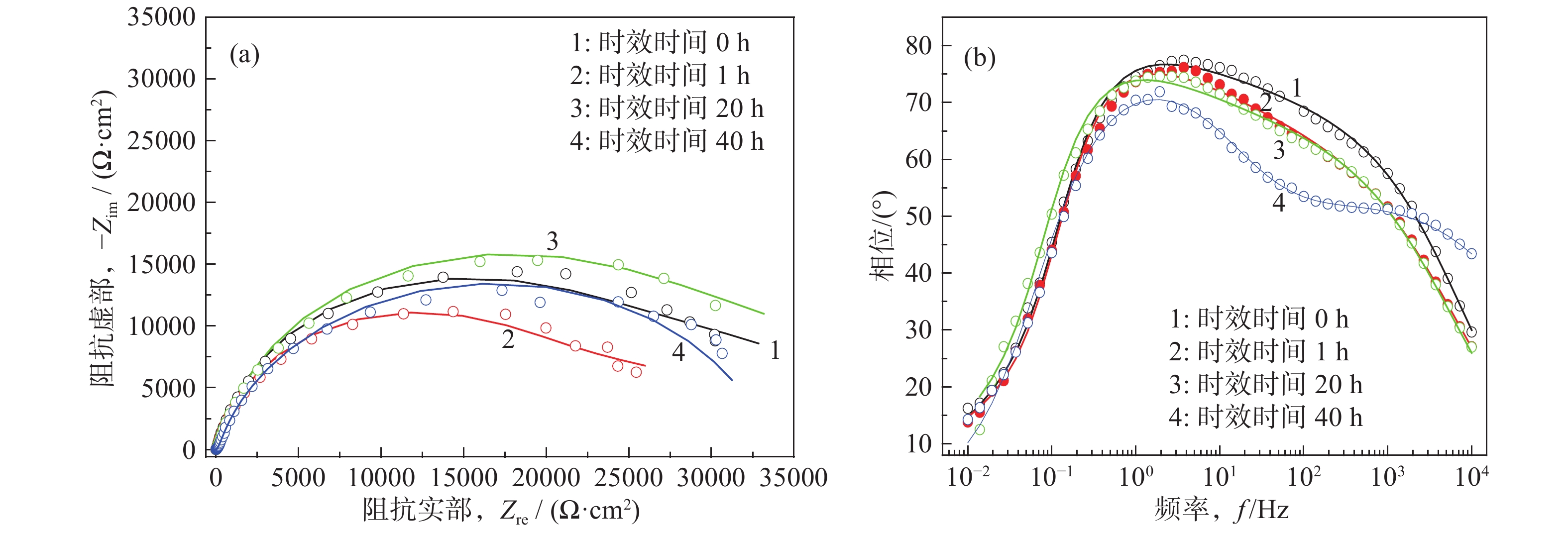
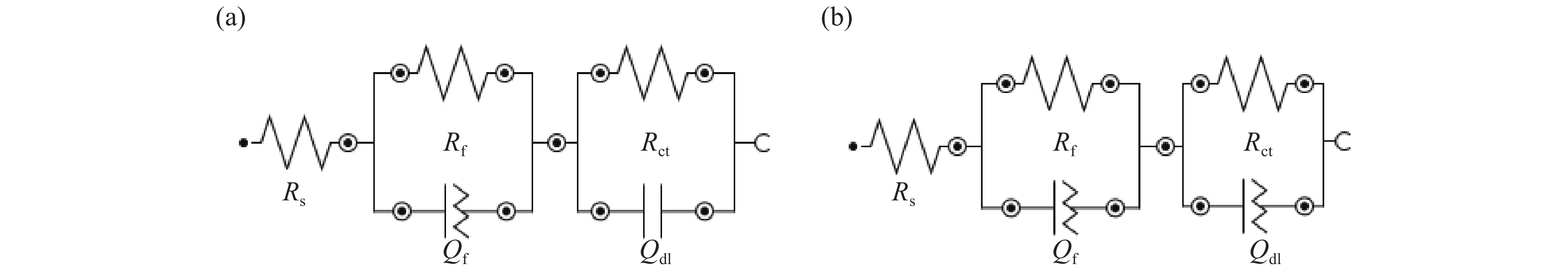
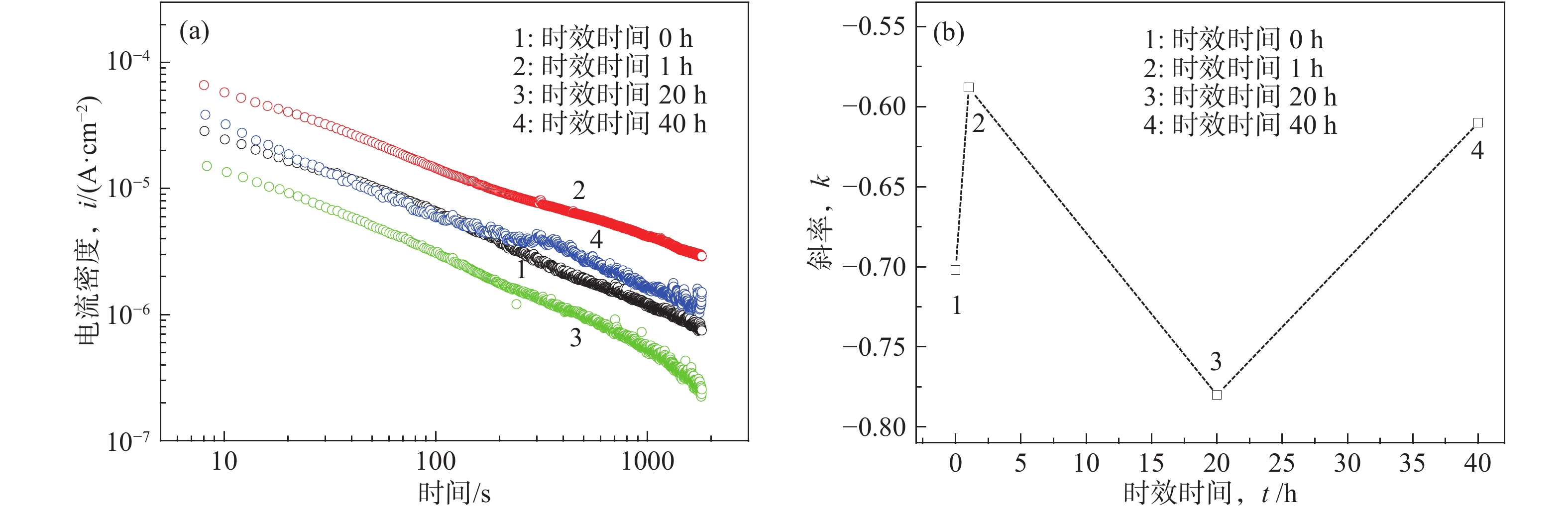
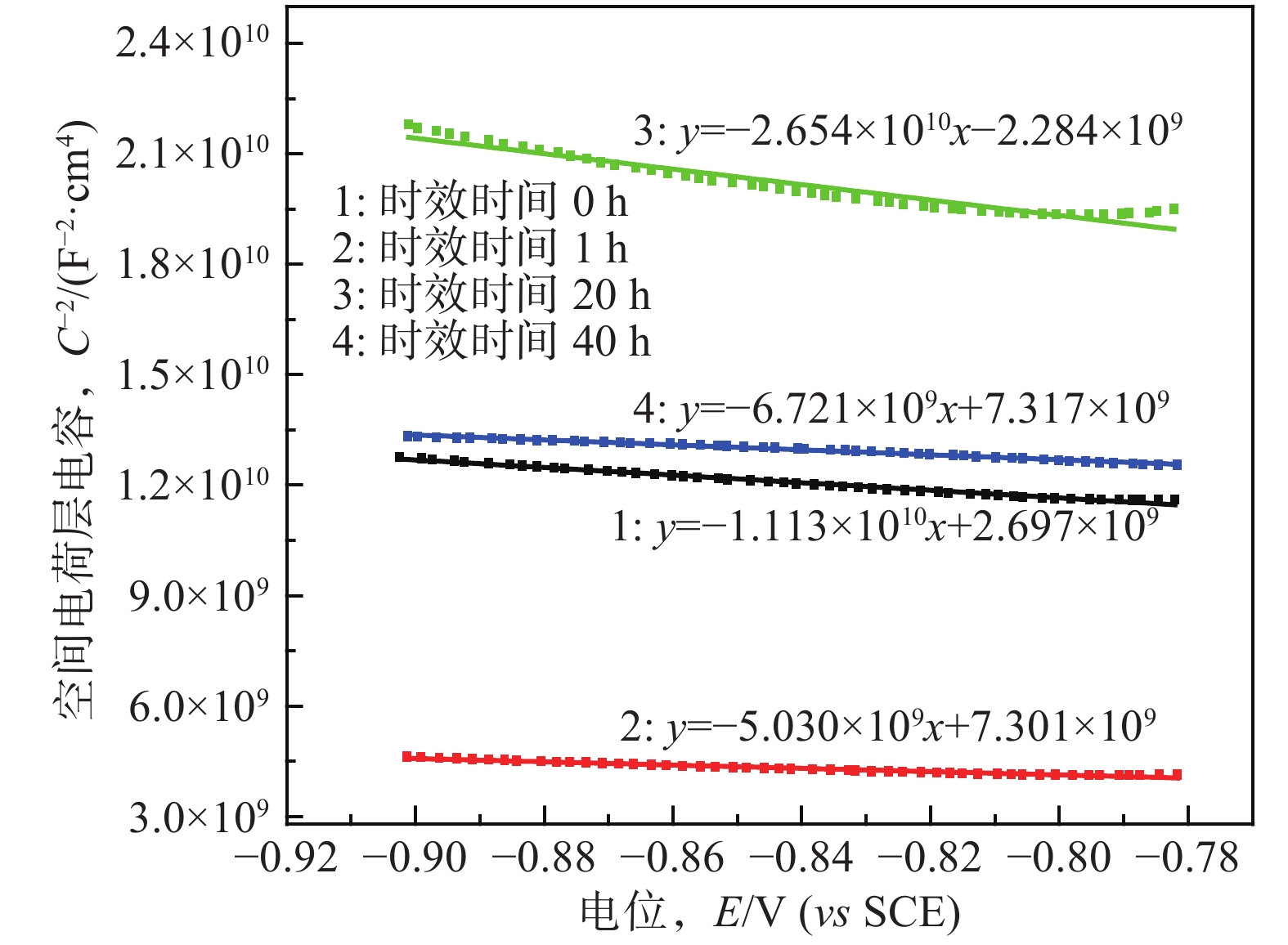
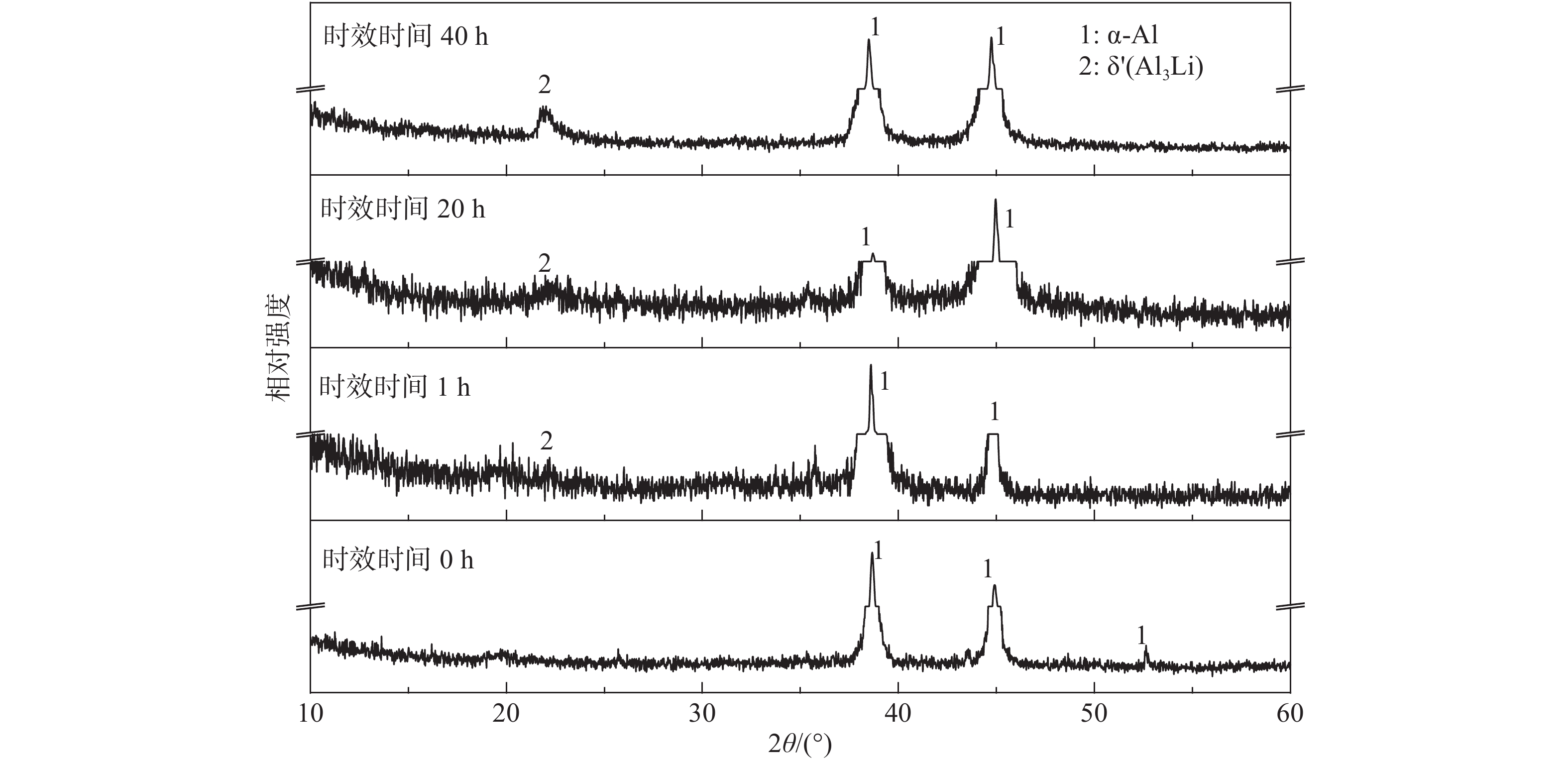
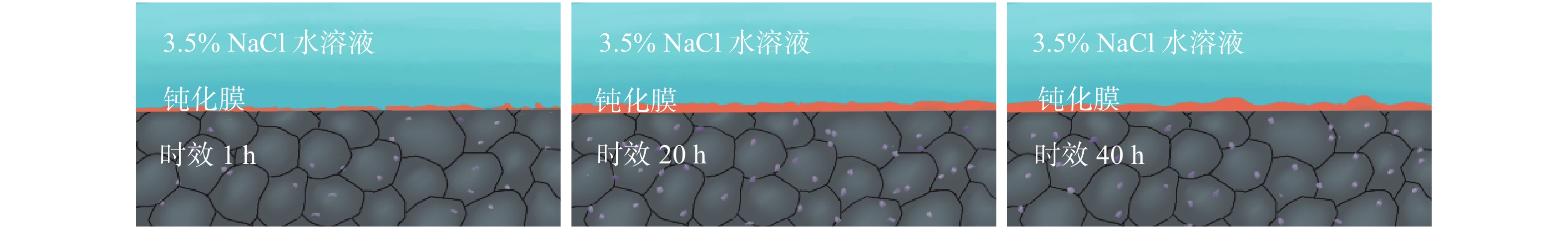
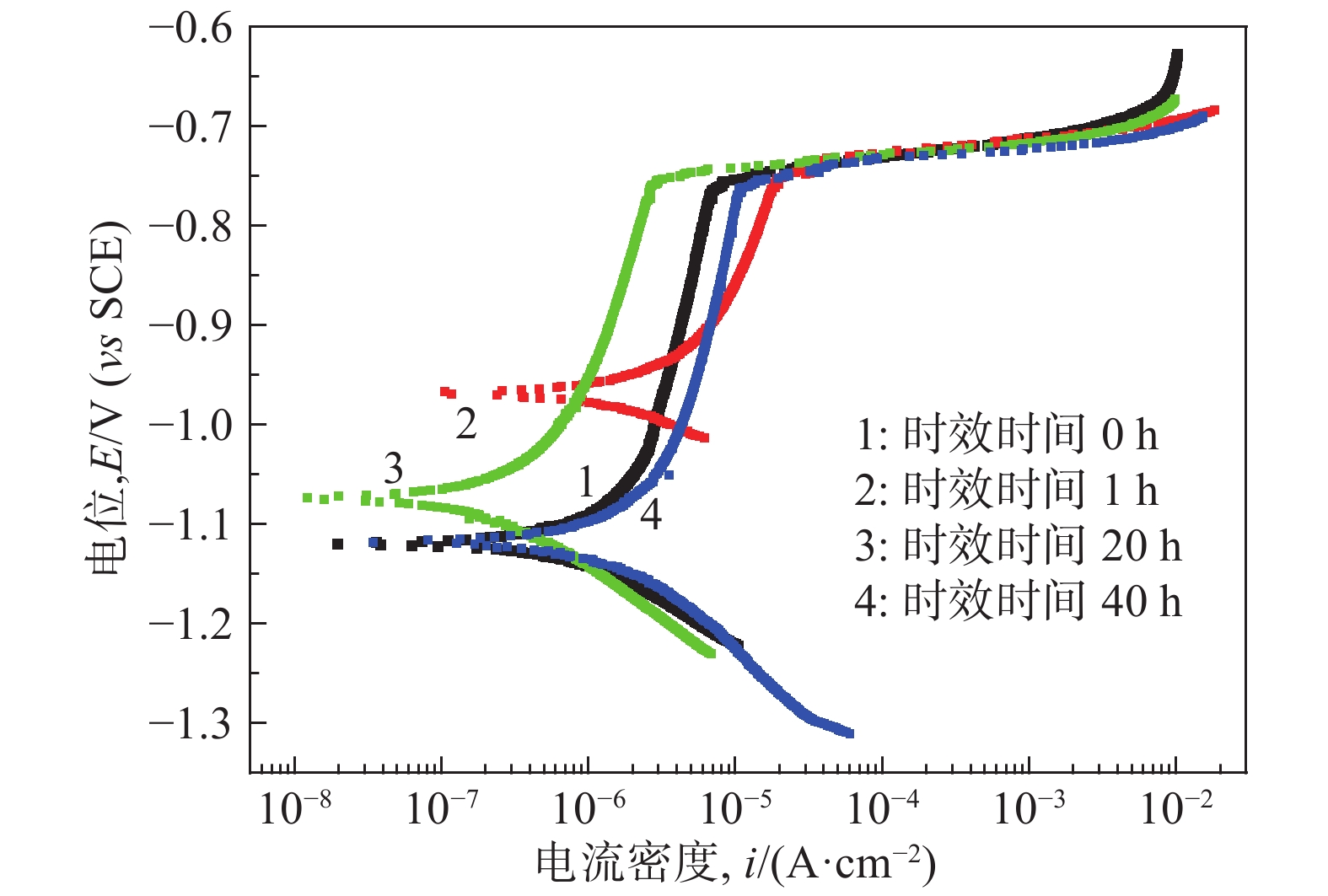
摘要: Al?Li合金具有低密度、高強韌性和低的腐蝕疲勞擴展速率的優點,在航空領域有著廣泛應用。Al3Li(δ′)相是Al?Li合金中主要強化相之一,因含有活性元素Li對該合金的腐蝕行為產生顯著影響。為明確δ′相在Al?Li合金電化學腐蝕中的作用,真空熔煉制備Al?2Li二元合金,固溶后進行180 ℃等溫時效,用X射線衍射(XRD)檢測合金的相組成。在質量分數為3.5% 的NaCl水溶液中,用動電位極化的方法測量了該合金的極化曲線。?0.85 V vs SCE鈍化電位下形成鈍化膜后,用電化學阻抗(EIS)檢驗鈍化膜的耐蝕性;用恒電位陽極極化和Mott?Schottky(M?S)曲線對該合金鈍化膜的結構進行分析。結果表明,Al?2Li合金的自腐蝕電位隨時效時間增加先正移后負移;固溶和時效合金鈍化膜的EIS都由兩個容抗弧組成,時效未改變鈍化膜的腐蝕機制;鈍化膜耐蝕性由高到低的順序為:時效20 h>固溶>時效40 h>時效1 h,且耐蝕性與其致密性及膜內的載流子密度有關。
-
關鍵詞:
- Al?2Li二元合金 /
- 時效 /
- 鈍化膜 /
- 耐蝕性 /
- 結構
Abstract: Al?Li alloys have the advantage of low density, high strength and toughness, and low corrosion fatigue rate. This combination of properties has led to their use in aerospace. Al3Li(δ′)phase is one of main strengthening phases of Al?Li alloys. The higher chemical reactivity of Li clearly influences its corrosion behavior. In order to explain the effect of δ′ phase in the Al?Li alloy electrochemical corrosion process, an Al?2Li binary alloy was prepared by vacuum melting. Aging treatment of Al?2Li alloy at 180 ℃ followed by solution treatment were carried out. A potentiodynamic polarization plot of the alloy was tested in a 3.5% (mass fraction) NaCl solution. Phase composition of all samples was determined by X-ray diffraction (XRD). Passive film was formed on this alloy at a passivation potential of ?0.85 V vs SCE. Corrosion resistance of the passive film on the surface of the Al?2Li binary alloy was tested by electrochemical impedance spectroscopy (EIS). Structure of the passive film was analyzed by potentiostatic polarization and the Mott?Schottky (M?S) approach. Results show that the corrosion potentials of Al?2Li alloy initially move toward the positive, then toward negative, along with increasing aging time. EIS spectra of the passive films on the solution treatment and aging have two capacitive impedance arcs; the corrosion mechanism is not changed by the aging treatment. Corrosion resistance of the passive film is, in order from high to low, aged 20 h > solution treatment > aged 40 h > aged 1 h, and is related to the compactness and acceptor concentration of the passive film.-
Key words:
- Al?2Li binary alloy /
- aging /
- passive film /
- corrosion resistance /
- structure
-
表 1 Al?2Li合金動電位極化曲線的擬合結果
Table 1. Fitting results of potentiodynamic polarization plots of the Al?2Li alloy
時效時間/h Ecorr/V(vs SCE) Icorr /(10?7·A·cm?2) Ep/(V vs SCE) 0 ?1.115 3.23 ?0.755 1 ?0.964 6.34 ?0.746 20 ?1.072 0.73 ?0.750 40 ?1.114 3.26 ?0.753 時效時間/h Rs/(Ω·cm2) Qf Rf/(kΩ·cm2) Cdl/(μF·cm?2) Rct/(kΩ·cm2) Y0/(Ω?1·cm?2·s?n) n 0 2.46 2.41×10?6 0.69 22.81 76.90 19.9 1 3.22 3.23×10?4 0.65 19.19 89.92 16.7 20 3.92 2.33×10?6 0.66 25.01 71.96 22.57 中文字幕在线观看時效時間/h Rs/(Ω·cm2) Qf Rf/(kΩ·cm2) Qdl Rct/(kΩ·cm2) Y0/(Ω?1·cm?2·s?n) n Y0/(Ω?1·cm?2·s?n) n 40 2.08 2.46×10?4 0.58 0.154 6.25×10?5 0.86 33.43 -
參考文獻
[1] Heinz A, Haszler A, Keidel C, et al. Recent development in aluminium alloys for aerospace applications. Mater Sci Eng A, 2000, 280(1): 102 doi: 10.1016/S0921-5093(99)00674-7 [2] Ovei H, Jagle E A, Stark A, et al. Microstructural influences on strengthening in a naturally aged and overaged Al?Cu?Li?Mg based alloy. Mater Sci Eng A, 2015, 637: 162 doi: 10.1016/j.msea.2015.04.039 [3] Li H Y, Huang D S, Kang W, et al. Effect of different aging processes on the microstructure and mechanical properties of a novel Al?Cu?Li alloy. J Mater Sci Technol, 2016, 32(10): 1049 doi: 10.1016/j.jmst.2016.01.018 [4] Yu X X, Yin D F, Yu Z M, et al. Effects of cerium addition on solidification behaviour and intermetallic structure of novel Al-Cu-Li alloys. Rare Met Mater Eng, 2016, 45(6): 1423 doi: 10.1016/S1875-5372(16)30125-4 [5] Yu X X, Yin D F, Yu Z M, et al. Microstructure evolution of novel Al?Cu?Li?Ce alloys during homogenization. Rare Met Mater Eng, 2016, 45(7): 1687 doi: 10.1016/S1875-5372(16)30141-2 [6] Willams D B, Edington J W. The discontinuous precipitation reaction in dilute Al?Li alloys. Acta Metall, 1976, 24(4): 323 doi: 10.1016/0001-6160(76)90007-9 [7] Baumann S F, Willams D B. A new method for the determination of the precipitate-matrix interfacial energy. Scripta Metall, 1984, 18(6): 611 doi: 10.1016/0036-9748(84)90351-X [8] Deschamps A, Sigli C, Mourey T, et al. Experimental and modelling assessment of precipitation kinetics in an Al?Li?Mg alloy. Acta Mater, 2012, 60(5): 1917 doi: 10.1016/j.actamat.2012.01.010 [9] Kolobney N I, Khokhlatova L B, Fridlyander I N. Aging of Al?Li alloys having composite particles of hardening phases. Mater Forum, 2004, 28: 208 [10] Pérez-Landazábal J I, Nó M L, Madariaga G, et al. Quantitative analysis of δ' precipitation kinetics in Al?Li alloys. Acta Mater, 2000, 48(6): 1283 doi: 10.1016/S1359-6454(99)00421-8 [11] Huang J C, Ardell A J. Precipitation strengthening of binary Al?Li alloys by δ' precipitates. Mater Sci Eng A, 1988, 104: 149 doi: 10.1016/0025-5416(88)90416-8 [12] Lewandowska M, Mizera J, Wyrzkowski J W. Cyclic behaviour of model Al?Li alloys: effect of the precipitate state. Mater Charact, 2000, 45(3): 195 doi: 10.1016/S1044-5803(00)00074-7 [13] Prasad K S, Mukhopadhyay A K, Gokhale A A, et al. δ precipitation in an Al?Li?Cu?Mg?Zr alloy. Scripta Metall Mater, 1994, 30(10): 1299 doi: 10.1016/0956-716X(94)90262-3 [14] Lin Y, Zheng Z Q, Li S C, et al. Microstructures and properties of 2099 Al?Li alloy. Mater Charact, 2013, 84: 88 doi: 10.1016/j.matchar.2013.07.015 [15] Chai C, Li J F, Wang H, et al. Dependence of intergranular corrosion sensitivity of Al?Li alloys on aging stage. Rare Met Mater Eng, 2015, 44(10): 2523蔡超, 李勁風, 王恒, 等. 鋁鋰合金晶間腐蝕敏感性與時效階段的相關性. 稀有金屬材料與工程, 2015, 44(10):2523 [16] Ma Y L, Zhou X R, Meng X M, et al. Influence of thermomechanical treatments on localized corrosion susceptibility and propagation mechanism of AA2099 Al?Li alloy. Trans Nonferrous Met Soc China, 2016, 26(6): 1472 doi: 10.1016/S1003-6326(16)64252-8 [17] Goebel J, Ghidini T, Graham A J. Stress-corrosion cracking characterization of the advanced aerospace Al?Li 2099-T86 alloy. Mater Sci Eng A, 2016, 673: 16 doi: 10.1016/j.msea.2016.07.013 [18] Niskanen P, Sanders T H. Influence of microstructure on the corrosion of Al?Li, Al?Li?Mn, Al?Li?Mg and Al?Li?Cu alloys in 3.5% NaCl solution. Bulletin de l'Association Technique Maritime et Aeronautique, 1981: 347 [19] Ambat R, Prasad R K, Dwarakadasa E S. The influence of aging at 180 ℃ on the corrosion behaviour of a ternary Al?Li?Zr alloy. Corros Sci, 1995, 37(8): 1253 doi: 10.1016/0010-938X(95)00030-N [20] Moreto J A, Marino C E B, Bose Filho W W, et al. SVET, SKP and EIS study of the corrosion behaviour of high strength Al and Al?Li alloys used in aircraft fabrication. Corros Sci, 2014, 84: 30 doi: 10.1016/j.corsci.2014.03.001 [21] Chao C Y, Lin L F, Macdonald D D. A point defect model for anodic passive films, I. Film growth kinetics. J Electrochem Soc, 1981, 128(6): 1187 doi: 10.1149/1.2127591 [22] Lin L F, Chao C Y, Macdonald D D. A point defect model for anodic passive films, Ⅱ. Chemical breakdown and pit initiation. J Electrochem Soc, 1981, 128(6): 1194 doi: 10.1149/1.2127592 [23] Morrison S R, translated by Wu H H. Electrochemistry at Semiconductor and Oxidized Metal Electrode. Beijing: Science Press, 1988 [24] Lü J L, Liang T X, Wang C, et al. The passive film characteristics of several plastic deformation 2099 Al?Li alloy. J Alloys Compd, 2016, 662: 143 doi: 10.1016/j.jallcom.2015.12.051 [25] Schultze J W, Lohrengel M M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim Acta, 2000, 45(15-16): 2499 doi: 10.1016/S0013-4686(00)00347-9 [26] Pletcher B A, Wang K G, Glicksman M E. Experimental, computational and theoretical studies of δ' phase coarsening in Al?Li alloys. Acta Mater, 2012, 60(16): 5803 doi: 10.1016/j.actamat.2012.07.021 [27] Chai Z G, Meng F L, Zou Q. The precipitation behiavior of δ' phase in Al?Li alloy treated by aging?retrogression?reaging. Acta Phys Sin, 2001, 50(7): 1401 doi: 10.3321/j.issn:1000-3290.2001.07.039柴志剛, 孟繁玲, 鄒青. Al?Li合金時效?回歸?再時效析出δ'相的行為. 物理學報, 2001, 50(7):1401 doi: 10.3321/j.issn:1000-3290.2001.07.039 -




 下載:
下載: