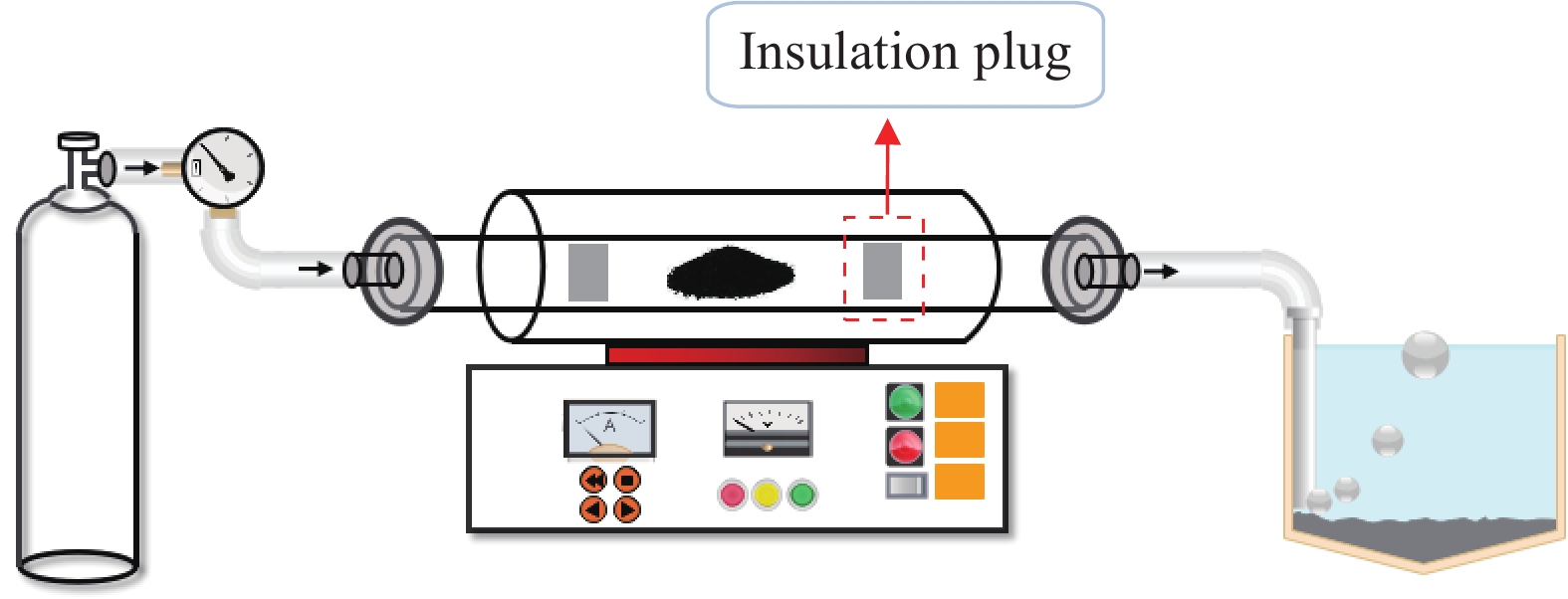
Preparation of metallic arsenic from calcium arsenate by carbon thermal roasting reduction
-
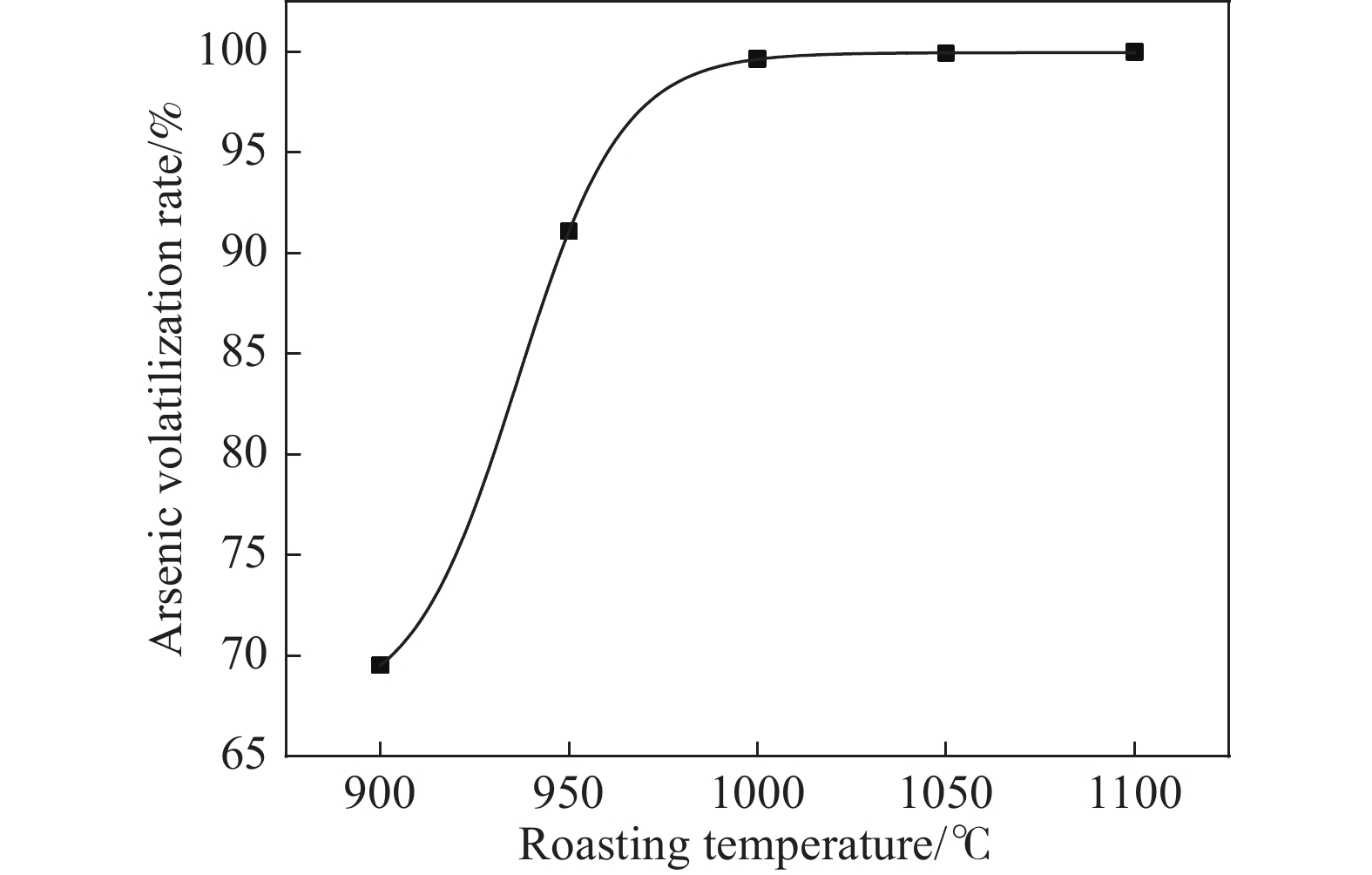
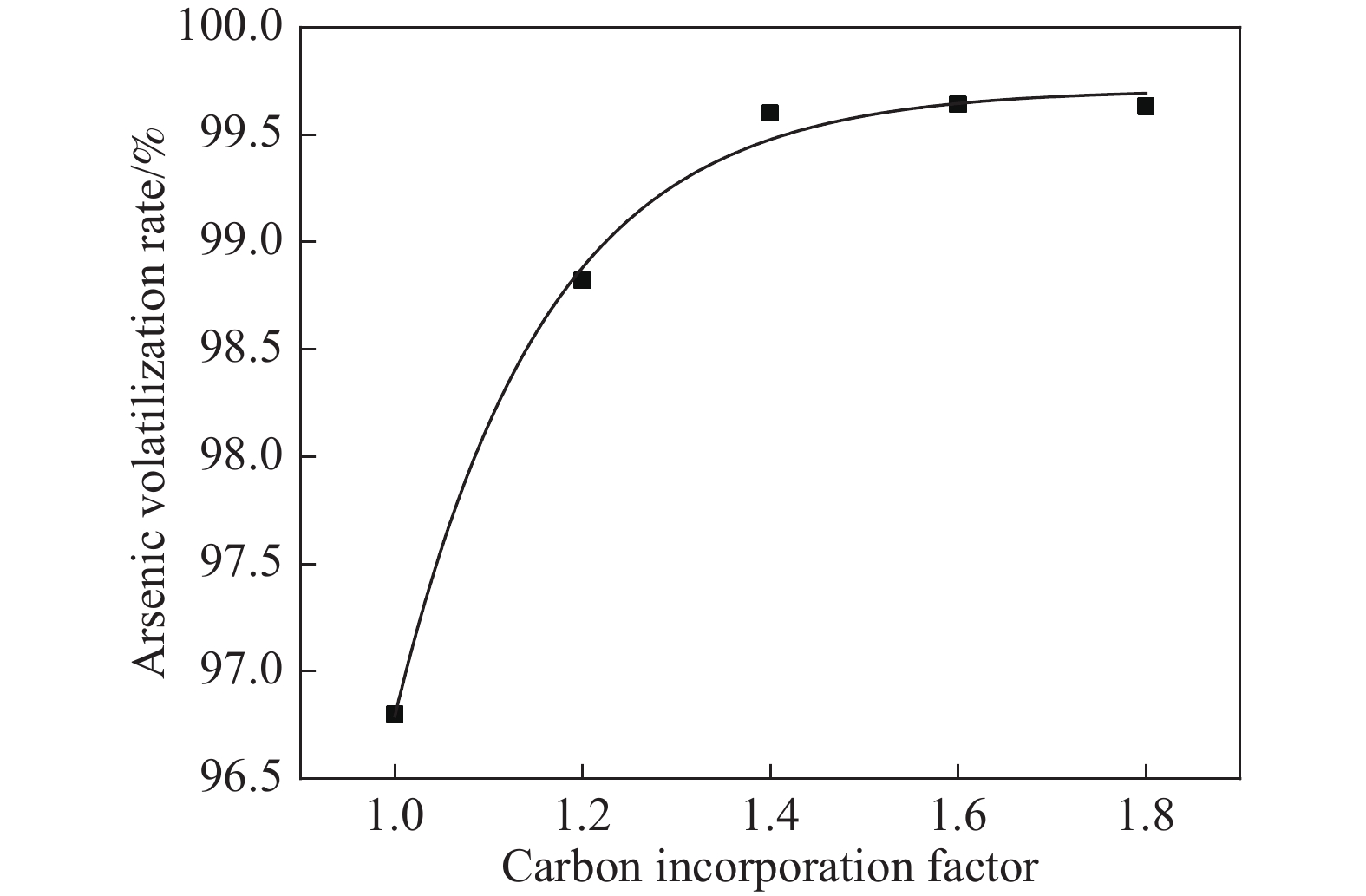
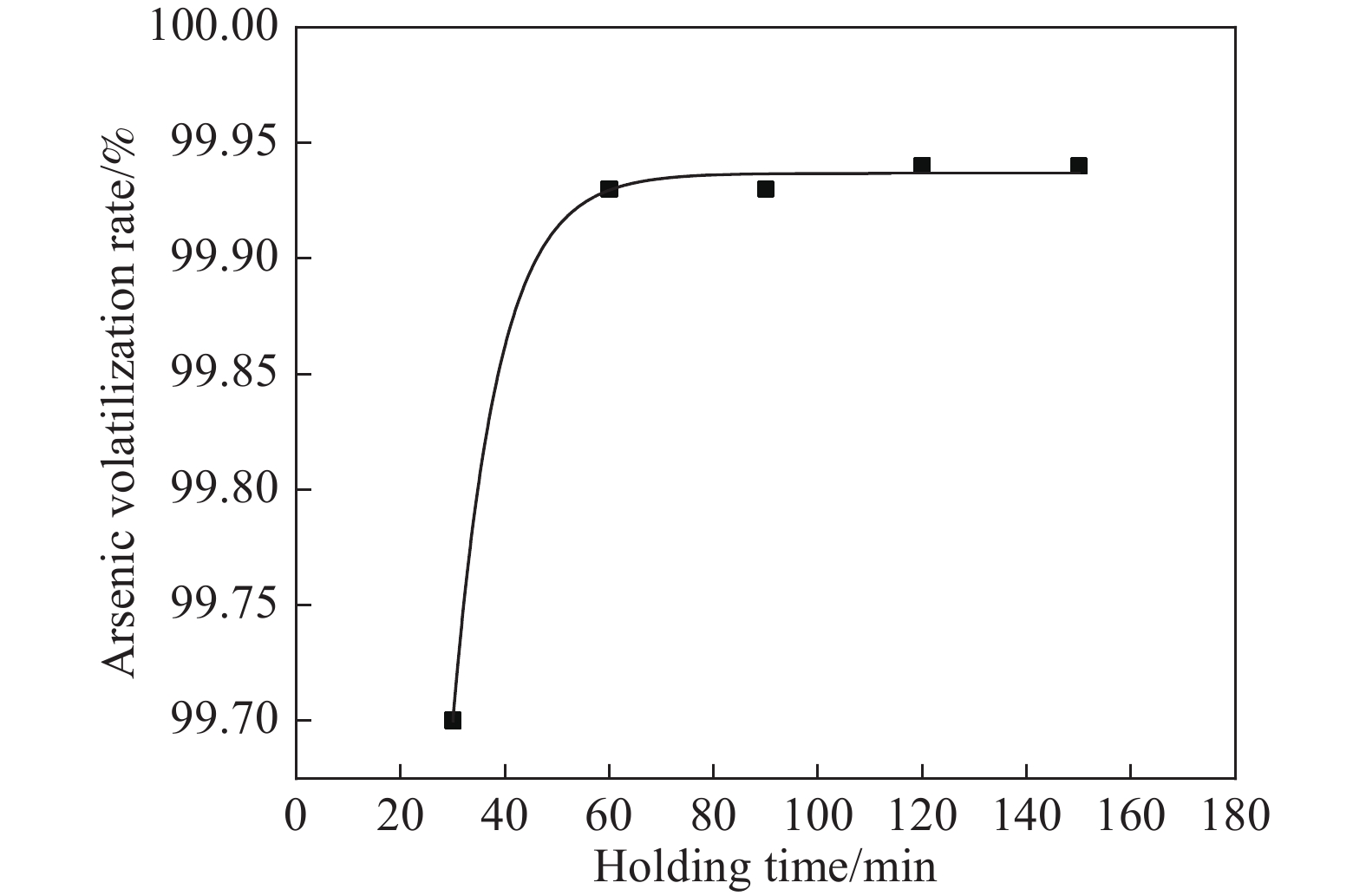
摘要: 致力于碳熱焙燒還原砷酸鈣制備具有商業價值的金屬單質砷,為推進砷危廢物無害化處理向砷資源化回收利用前進展開科學研究。其中熱重分析表明,砷酸鈣與碳粉混合熱解的質量損失分為3個階段,階段1和階段2為失水過程,階段3為碳還原砷酸鈣生成CaO和砷蒸氣過程。且研究發現,可以利用相邊界反應動力學模型解釋階段3反應機制。而單因素條件實驗結果表明:在溫度1000 ℃、碳配入系數1.4、恒溫時長60 min條件下砷揮發率高達99.94%。X射線衍射儀(XRD)、掃描電鏡能譜儀(SEM?EDS)對反應體系中有關產物表征表明,較優條件下產品砷主要為片狀金屬砷和粉末非晶體砷,焙燒殘渣為CaO。Abstract: Given the widespread application of the lime precipitation process for arsenic removal in the smelting of arsenic-containing minerals, the resourcefulness of calcium arsenate use has received increasing attention. In general, more types of arsenate have different high-temperature characteristics, and the slag type is complicated under mixed reduction roasting and difficult to recover. Additionally, arsenate in the form of calcium arsenate is a more common and inexpensive product in the metallurgical process. Because whether it is arsenic-containing wastewater, arsenic slag, arsenate, and so on, the material can be separated from the system by inexpensive lime precipitation or calcification transformation in simple metallurgical equipment to generate calcium arsenate. Therefore, this paper was devoted to preparing commercially valuable metallic monomers of arsenic by carbon thermal roasting reduction of calcium arsenate and to starting scientific research to advance the harmless treatment of arsenic hazardous waste to arsenic resource recovery and use. Among them, thermogravimetric analysis shows that the mass loss of calcium arsenate mixed with carbon powder pyrolysis is divided into 3 stages: stages 1 and 2 are water loss processes, and stage 3 involves the carbon reduction of calcium arsenate to generate CaO and arsenic vapor. It is found that the stage III reaction mechanism could be explained using the phase boundary reaction kinetic model. The experimental results of single-factor conditions show that the arsenic volatilization rate reaches 99.94% at a constant temperature of 1000 °C for 60 min and a carbon allotment factor of 1.4. The characterization of the relevant products in the reaction system by X-ray diffractometer (XRD) and scanning electron microscope energy spectrometer (SEM?EDS) show that the arsenic product is mainly flaked metallic arsenic and amorphous powdered arsenicunder better conditions, and the roasted residue is CaO.
-
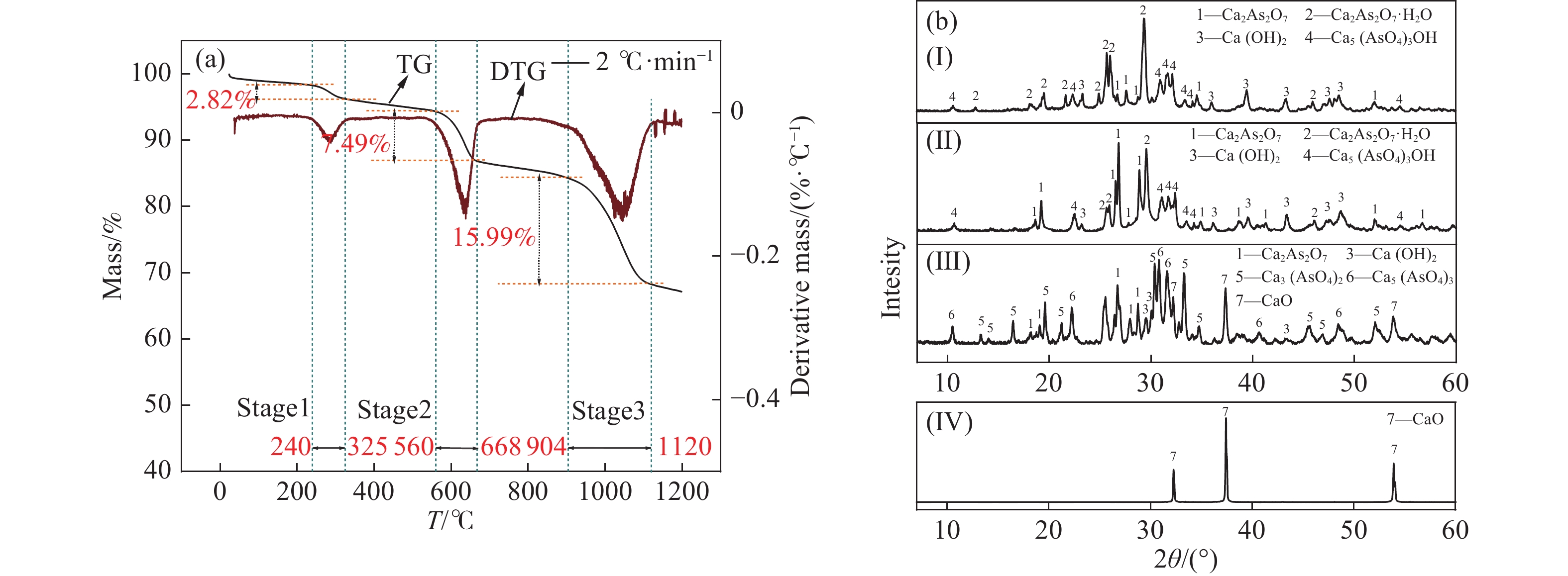
圖 3 砷酸鈣與碳粉混合熱解特性。(a)砷酸鈣與碳粉混合熱解的熱重?熱重微商曲線;(b)殘渣X射線衍射圖(Ⅰ原物料;Ⅱ第1質量損失階段,320 ℃;Ⅲ第2質量損失階段,700 ℃;Ⅳ第3質量損失階段,1000 ℃)
Figure 3. Pyrolysis characteristics of calcium arsenate mixed with carbon powder: (a) TG?DTG curves of the pyrolysis of calcium arsenate mixed with carbon powder; (b) XRD plots of the residue (Ⅰ raw material; Ⅱfirst mass loss stage, 320 ℃; Ⅲ second mass loss stage, 700 ℃; Ⅳ third mass loss stage, 1000 ℃)
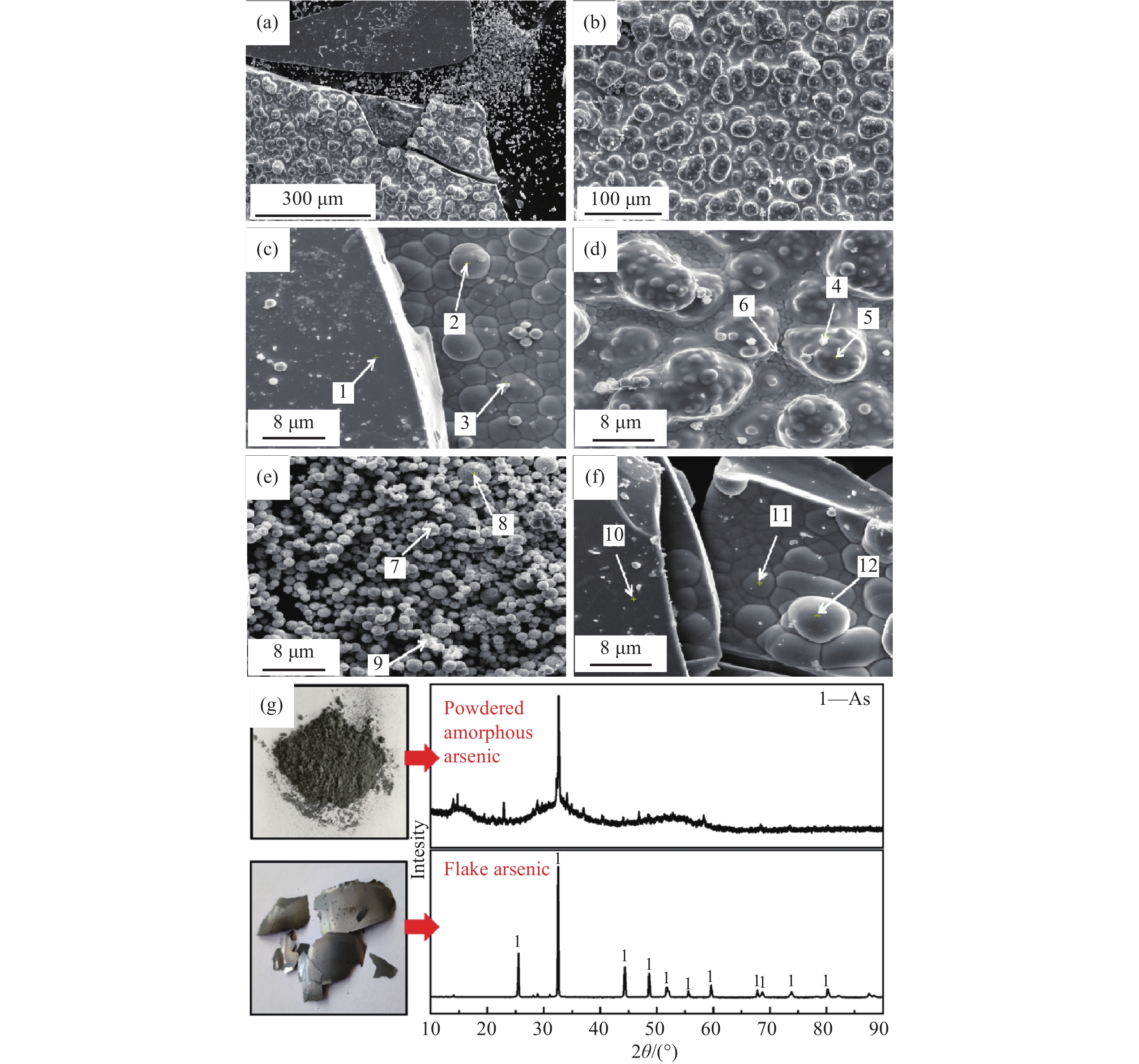
圖 8 產品砷掃描電鏡和X射線衍射圖譜。(a~c)不同放大倍數金屬片砷;(d)粗糙反面金屬片砷;(e)粉末不定型砷;(f)光澤正面金屬片砷;(g) 產品砷X射線衍射圖
Figure 8. Arsenic product SEM, XRD patterns: (a?c) metal flake arsenic with different magnification; (d) rough back side of the metal flake arsenic; (e) powdered unshaped arsenic; (f) glossy front side of the metal flake arsenic; (g) XRD of arsenic product
表 1 合成砷酸鈣的主要成分(質量分數)
Table 1. Main components of synthetic calcium arsenate
% Ca As O Other 19.81 31.43 38.41 10.35 表 2 動力學機理函數
Table 2. Kinetic mechanism function
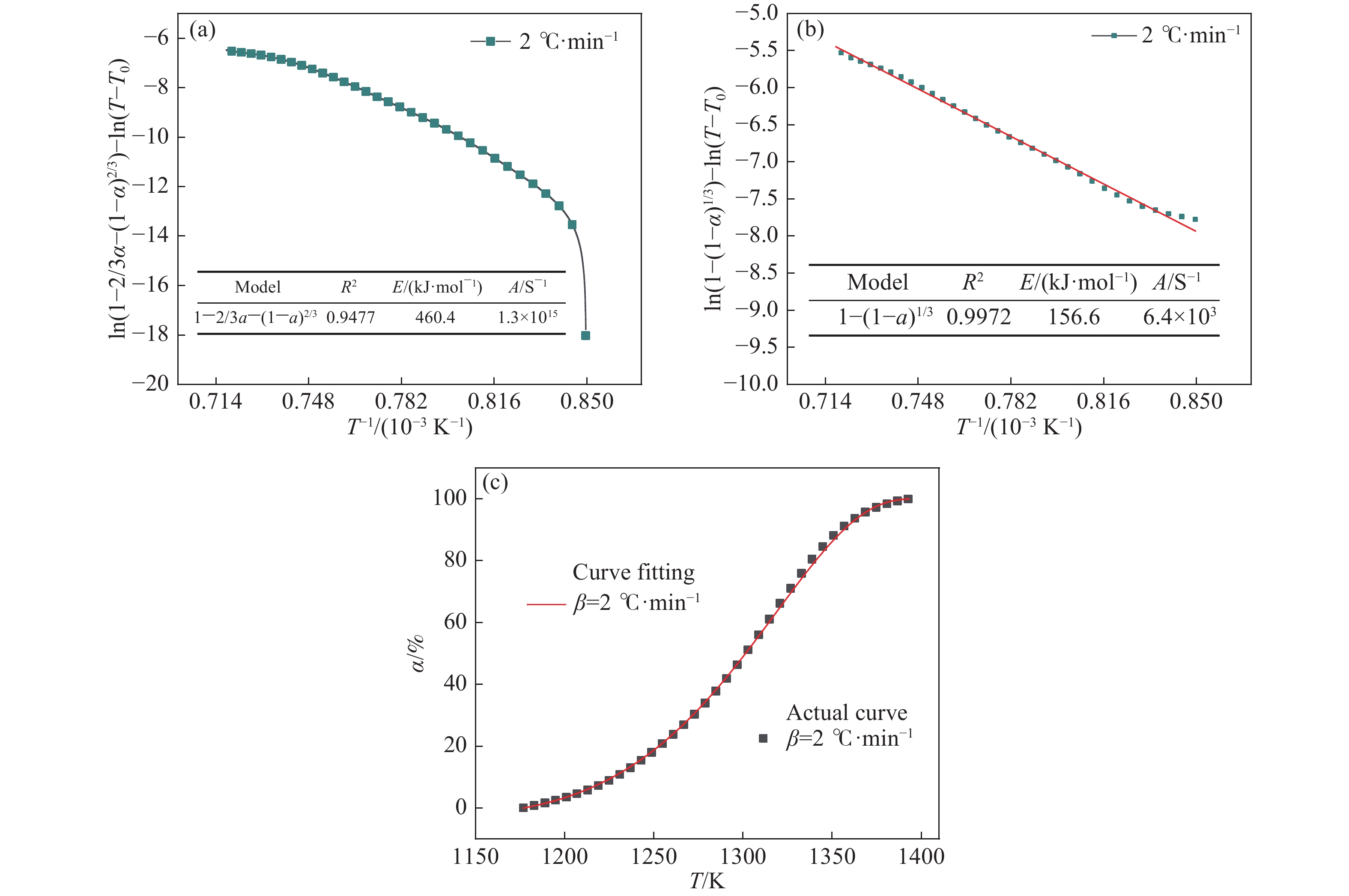
Function number Function name Mechanism Points form G(α) 1 Ginstling?Brounshteine
equationThree-dimensional diffusion $ 1-\dfrac{2}{3}\alpha -(1-\alpha {)}^{\frac{2}{3}} $ 2 Shrink globular Phase boundary reaction $ 1-(1-\alpha {)}^{\frac{1}{3}} $ 中文字幕在线观看表 3 金屬砷的掃描電鏡能譜分析結果(質量分數)
Table 3. Results of EDS analysis of arsenic metal
% number O As 1 1.16 98.84 2 0.85 99.15 3 2.44 97.55 4 23.35 76.65 5 0.72 99.28 6 0.92 99.08 7 1.63 98.37 8 1.14 98.86 10 0.48 99.52 11 1.32 98.68 12 0.38 99.62 -
參考文獻
[1] Jr J V B, Brown P W. The stabilities of calcium arsenates at 23±1 ℃. J Hazard Mater, 1999, 69(2): 197 doi: 10.1016/S0304-3894(99)00105-3 [2] Long G, Peng Y J, Bradshaw D. A review of copper-arsenic mineral removal from copper concentrates. Miner Eng, 2012, 36-38: 179 doi: 10.1016/j.mineng.2012.03.032 [3] Lee P K, Yu S, Jeong Y J, et al. Source identification of arsenic contamination in agricultural soils surrounding a closed Cu smelter, South Korea. Chemosphere, 2019, 217: 183 doi: 10.1016/j.chemosphere.2018.11.010 [4] Leist M, Casey R J, Caridi D. The fixation and leaching of cement stabilized arsenic. Waste Manag, 2003, 23(4): 353 doi: 10.1016/S0956-053X(02)00116-2 [5] Yuan Y F, Liu S H. Distribution and removal of arsenic in the process of bottom-blowing continuous copper smelting. China Nonferrous Metall, 2020, 49(2): 37袁永鋒, 劉素紅. 底吹連續煉銅過程中砷的走向及控制. 中國有色冶金, 2020, 49(2):37 [6] Jia H. Recycling and Comprehensive Utilization of Metallurgical Waste with High Arsenic Content [Dissertation]. Changsha: Central South University, 2013賈海. 高砷冶金廢料的回收與綜合利用[學位論文]. 長沙: 中南大學, 2013 [7] Liu G, Shi Y, Guo G L, et al. Soil pollution characteristics and systemic environmental risk assessment of a large-scale arsenic slag contaminated site. J Clean Prod, 2020, 251: 119721 doi: 10.1016/j.jclepro.2019.119721 [8] Dutré V, Vandecasteele C. Solidification/stabilisation of arsenic-containing waste: Leach tests and behaviour of arsenic in the leachate. Waste Manag, 1995, 15(1): 55 doi: 10.1016/0956-053X(95)00002-H [9] Dutré V, Vandecasteele C. Solidification/stabilisation of hazardous arsenic containing waste from a copper refining process. J Hazard Mater, 1995, 40(1): 55 doi: 10.1016/0304-3894(94)00080-Z [10] Dutré V, Vandecasteele C. An evaluation of the solidification/stabilisation of industrial arsenic containing waste using extraction and semi-dynamic leach tests. Waste Manag, 1996, 16(7): 625 doi: 10.1016/S0956-053X(97)00003-2 [11] Vandecasteele C, Dutré V, Geysen D, et al. Solidification/stabilisation of arsenic bearing fly ash from the metallurgical industry. Immobilisation mechanism of arsenic. Waste Manag, 2002, 22(2): 143 [12] Choi W H, Lee S R, Park J Y. Cement based solidification/stabilization of arsenic-contaminated mine tailings. Waste Manag, 2009, 29(5): 1766 doi: 10.1016/j.wasman.2008.11.008 [13] Akhter H, Cartledge F K, Roy A, et al. Solidification/stabilization of arsenic salts: Effects of long cure times. J Hazard Mater, 1997, 52(2-3): 247 doi: 10.1016/S0304-3894(96)01811-0 [14] Singh T S, Pant K K. Solidification/stabilization of arsenic containing solid wastes using Portland cement, fly ash and polymeric materials. J Hazard Mater, 2006, 131(1-3): 29 doi: 10.1016/j.jhazmat.2005.06.046 [15] Yoon I H, Moon D H, Kim K W, et al. Mechanism for the stabilization/solidification of arsenic-contaminated soils with Portland cement and cement kiln dust. J Environ Manag, 2010, 91(11): 2322 doi: 10.1016/j.jenvman.2010.06.018 [16] Zhao Z W, Song Y X, Min X B, et al. XPS and FTIR studies of sodium arsenate vitrification by cullet. J Non Cryst Solids, 2016, 452: 238 doi: 10.1016/j.jnoncrysol.2016.08.028 [17] Zhao Z W, Chai L Y, Peng B, et al. Arsenic vitrification by copper slag based glass: Mechanism and stability studies. J Non Cryst Solids, 2017, 466-467: 21 doi: 10.1016/j.jnoncrysol.2017.03.039 [18] Xu J B, Shen Q H, Chen W, et al. The present situation and the countermeasure of the processing of arsenic residues. Min Metall, 2017, 26(3): 82 doi: 10.3969/j.issn.1005-7854.2017.03.018徐建兵, 沈強華, 陳雯, 等. 含砷廢渣處理現狀及對策. 礦冶, 2017, 26(3):82 doi: 10.3969/j.issn.1005-7854.2017.03.018 [19] Lu X Y. The status quo and progress of arsenic-containing waste residue treatment technology. Mod Salt Chem Ind, 2018, 45(5): 87 doi: 10.3969/j.issn.1005-880X.2018.05.041陸曉陽. 含砷廢渣處理技術的現狀與進展. 現代鹽化工, 2018, 45(5):87 doi: 10.3969/j.issn.1005-880X.2018.05.041 [20] Lu H B. Thermodynamic analysis on crude metal arsenic preparation by arsenic trioxide carbothermic reduction in vacuum. Nonferrous Met (Extr Metall) , 2012(10): 55盧紅波. As2O3真空碳熱還原制備粗金屬砷的熱力學研究. 有色金屬(冶煉部分), 2012(10):55 [21] Li X P. Experiment study on arsenic preparation by dc furnace. Min Metall, 2012, 21(3): 56 doi: 10.3969/j.issn.1005-7854.2012.03.015李學鵬. 直流電弧爐制備金屬砷試驗研究. 礦冶, 2012, 21(3):56 doi: 10.3969/j.issn.1005-7854.2012.03.015 [22] Pan C F. An effective way to improve the quality of metallic arsenic. Nonferrous Met (Extr Metall) , 1994(2): 32潘崇發. 提高金屬砷質量的有效途徑. 有色金屬(冶煉部分), 1994(2):32 [23] Huang Z L, Liu Y Y, Tao Q Y, et al. Influencing factors of arsenic removal by lime precipitation. Chin J Environ Eng, 2012, 6(3): 734黃自力, 劉緣緣, 陶青英, 等. 石灰沉淀法除砷的影響因素. 環境工程學報, 2012, 6(3):734 [24] Zhang H. Study on the solubility and stability of calcium arsenate [Dissertation]. Guilin: Guilin Institute of Technology, 2005張華. 砷酸鈣鹽的溶解度和穩定性研究[學位論文]. 桂林: 桂林工學院, 2005 [25] Liu H L, Zhu Y N. Thermodynamic analysis of the CO2 effects on the stability of calcium arsenates. Environ Prot Sci, 2006, 32(3): 7 doi: 10.3969/j.issn.1004-6216.2006.03.003劉輝利, 朱義年. CO2對砷酸鈣穩定性影響的熱力學分析. 環境保護科學, 2006, 32(3):7 doi: 10.3969/j.issn.1004-6216.2006.03.003 [26] Bothe J V, Brown P W. Arsenic immobilization by calcium arsenate formation. Environ Sci Technol, 1999, 33(21): 3806 doi: 10.1021/es980998m [27] Wang G, Xue Q G, Shen Y F, et al. Carbothermic reduction kinetics of boron-bearing iron concentrate. Chin J Eng, 2016, 38(5): 623王廣, 薛慶國, 沈穎峰, 等. 硼鐵精礦的碳熱還原動力學. 工程科學學報, 2016, 38(5):623 [28] Hu R Z, Gao S L, Zhao F Q. Thermal Analysis Kinetics. 2nd ed. Beijing: Science Press, 2008: 20胡榮祖, 高勝利, 趙鳳起. 熱分析動力學. 2版. 北京: 科學出版社, 2008: 20 -




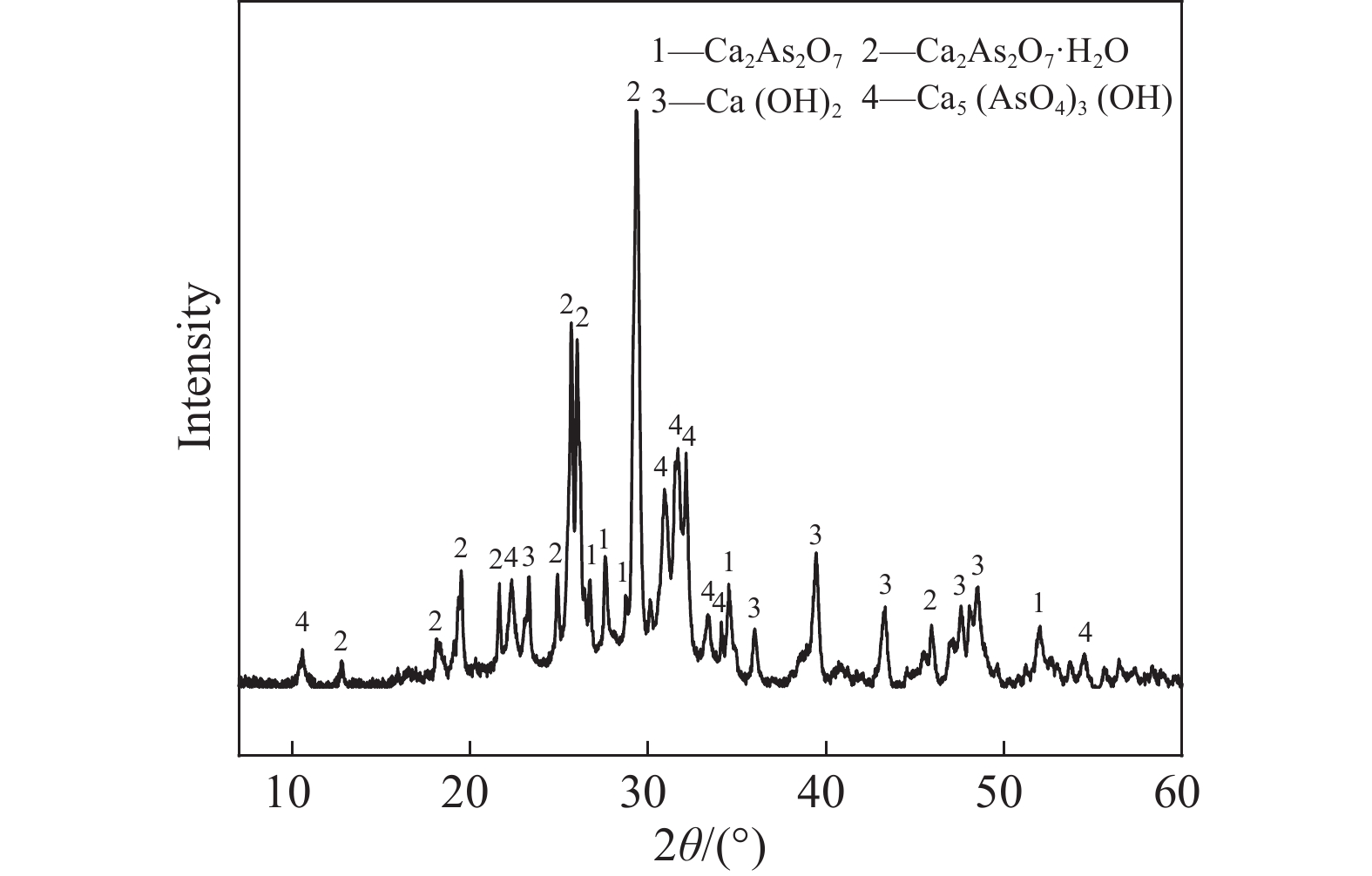
 下載:
下載: